Phosphoric acid equipment manufacturing
Phosphoric acid equipment production is used in many areas, especially fertilizer, pharmaceutical industry, petroleum industry, pesticide production and various cleaning chemicals.
- Home
- Fields-of-activity
- Phosphoric acid equipment manufacturing
Our fields of activity
- Fertilizer (Dap)
- Sulfuric acid
- Phosphoric acid
- Ammonia plant
- Cement factory
- Sugar factory
- Feed factory
- Power plant
- Hydroelectric plant
- Process equipment
- Chemical factory
- Pharmaceutical factory
- Oil factory
- Industrial facility construction
- Structural steel fabrication
- Hydrogen Peroxide
- Propane Dehydrogenation
- Equipment design
Promotional brochure
Download our catalogue to see specific data about the service we provide and how we work.
Stay in touch!
Please feel free to contact us. We will get back to you within 1-2 business days. Or call us now.
Phosphoric acid
Phosphoric acid provides corrosion resistant process equipment that is part of the pre-treatment (concentration) and final treatment (defluoration) of wet phosphoric acid production. There is more than one production method for phosphoric acid. The production of this chemical depends on the area in which it will be used.
Pure phosphoric acid is obtained by burning phosphorus in a mixture of air and steam. In case of production for use in fertilizers, it is aimed to obtain low purity phosphoric acid. We offer you reliable and quality service with our wide range of products consisting of plate heat exchangers, mixers and other flow equipment in phosphoric acid production processes.
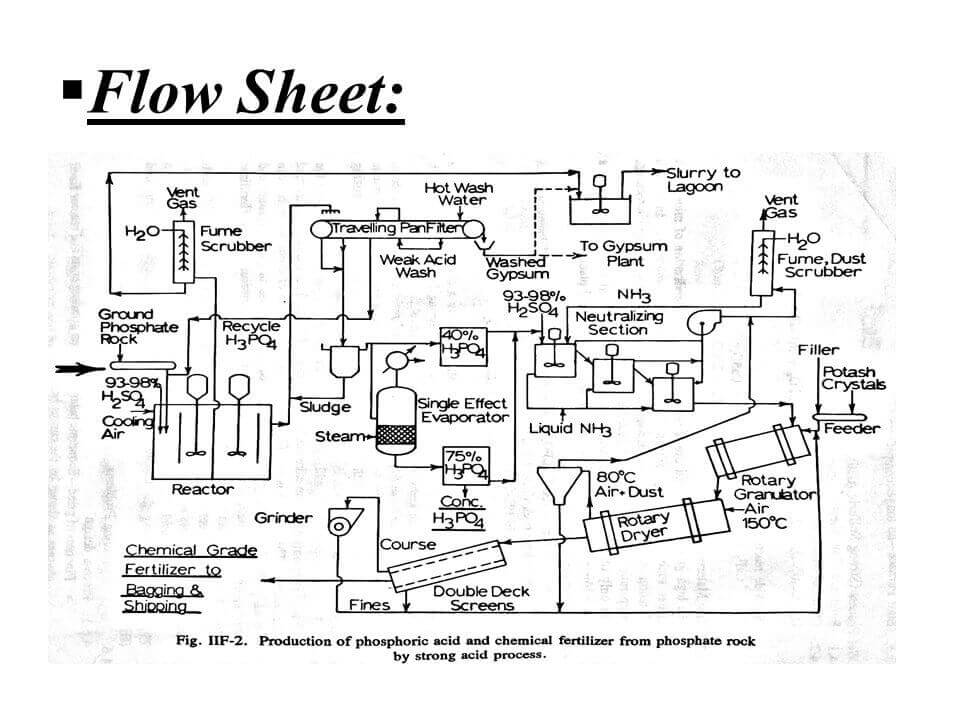
Phosphoric acid flow chart
The phosphoric acid wet process accounts for approximately 95% of world production. In the wet process, sulfuric acid (H2SO4) reacts with natural phosphate rocks to produce H3PO4 and calcium sulfate (CaSO4), commonly known as gypsum. The products from the phosphoric acid wet process are primarily used in fertilizer production because they are contaminated with impurities such as sulfates, fluorides and fluosilicates.
There are several types of wet process, the main differences being the operating temperature, with higher temperatures being more demanding on the materials used. The primary byproduct of the phosphoric acid production process is calcium sulfate, commonly known as gypsum. During the wet process production of phosphoric acid, sulfuric acid reacts with phosphate rock to produce phosphoric acid and gypsum.
Alloy selection criteria for phosphoric acid
Wet process phosphoric acid (WPA) can be aggressive to stainless steels depending on the range of impurities contained in the acid. This is of particular concern in bulk handling and transport of crude phosphoric acid. Most applications are based on stainless steel or nickel alloys. Plain chromium grades are generally unsuitable, but higher levels of chromium and molybdenum increase phosphoric acid resistance.
Any increase in temperature and/or the presence of chloride, fluoride and sulphuric acid impurities increases the risk of corrosion. Steel grades that are more resistant to pitting should be considered when these impurities are known to be present and specialist advice is needed to optimise grade selection.
Alloy 400
Nickel-copper class alloy.
Provides significant resistance to pure phosphoric acid, with corrosion rates of less than 0.05 mm/y at 10-100% concentrations up to 80oC
Corrosion will increase significantly at higher temperatures and will become significant with the addition of oxidizing salts.
Alloy 600
Nickel-chromium alloy.
Resistant to phosphoric acid at room temperature, but corrosion rates increase significantly with temperature.
Alloy 625
Nickel-chromium-molybdenum-niobium class alloy. Superior resistance in the presence of significant chlorides and fluoride.
Alloy 625 provides excellent performance in 25% phosphoric acid solution at the boiling point.
Real world tests show nominal rates and no localized corrosion in evaporation of wet process phosphoric acid.
Alloy C276
Nickel-molybdenum-chromium alloy obtained by solution annealing with the addition of tungsten.
The most widely used corrosion resistant material today.
The high molybdenum and chromium content allows the alloy to perform in oxidizing, non-oxidizing and mixed acid environments, while exhibiting exceptional resistance to pitting and crevice corrosion attack.
Alloy 825
Nickel-iron-chromium grade with molybdenum and copper additions.
Alloy 825 provides excellent resistance to reducing environments such as those containing sulfuric and phosphoric acids.
Molybdenum prevents pitting and crevice corrosion.
The chromium content of the alloy increases resistance to various oxidizing agents such as nitric acid, nitrates and oxidizing salts.
Significant exposure to chlorides and fluorides may cause pitting of alloy 825.
Alloy AL-6XN®
Super-austenitic nickel-chromium-molybdenum-nitrogen stainless steel.
AL-6XN® alloy is resistant to phosphoric acid concentrations above 45%.
In a boiling 20% phosphoric acid solution, the corrosion rate of AL-6XN is 0.006 mm/y, compared to alloy 316L, for example, which has a corrosion rate of 0.02 mm/y.
Alloy 316L
Austenitic chromium-nickel-molybdenum stainless steel.
Excellent resistance to phosphoric acid at 40% concentrations, good resistance at 40%-100% concentrations.
Corrosion will increase in the presence of chloride or fluoride.