Sulfuric acid factory equipment manufacturing
Integrated Sulfuric acid factory equipment manufacturing provides savings and improved environmental performance.
- Home
- Fields-of-activity
- Sulfuric acid factory equipment manufacturing
Our fields of activity
- Fertilizer (Dap)
- Sulfuric acid
- Phosphoric acid
- Ammonia plant
- Cement factory
- Sugar factory
- Feed factory
- Power plant
- Hydroelectric plant
- Process equipment
- Chemical factory
- Pharmaceutical factory
- Oil factory
- Industrial facility construction
- Structural steel fabrication
- Hydrogen Peroxide
- Propane Dehydrogenation
- Equipment design
Promotional brochure
Download our catalogue to see specific data about the service we provide and how we work.
Stay in touch!
Please feel free to contact us. We will get back to you within 1-2 business days. Or call us now.
Sulfuric acid factory equipment manufacturing
Internally produced acid closes the mill’s Na-S chemical balance. Sodium and Sulfur are essential chemical elements in a kraft pulp mill and play a vital role in the production of quality pulp. The balance of these chemicals is directly dependent on the efficiency of the pulp mill. Sulfur odorous gases reduced in the pulp mill can be converted to sulfuric acid, and this internally produced acid closes the chemical circulation, providing economic and environmental benefits.
Sulfuric acid
Concentrated non-condensable gases (CNCG) are collected and sent to a collection tank, from where they are taken to a separate incinerator. In the separate incinerator, the total reduced sulfur compounds in the CNCG are oxidized to SO2. The flue gases exiting the boiler are directed to the catalytic reaction vessel. The catalyst oxidizes SO2 to SO3 using excess oxygen provided by the combustion air. The flue gases exiting the catalytic converter are transferred to the condensation tower. The temperature of the flue gases is reduced by the cooling liquid, and the SO3 in the flue gases reacts with H2O to produce sulfuric acid.
Sulfuric acid plant equipment manufacturing is a critical stage used in the production process of sulfuric acid, one of the basic components of the chemical industry. This process involves the design, manufacturing and assembly of equipment with high quality standards. Equipment manufacturing consists of reactors, heating and cooling systems, pumps and piping systems. Each of these equipments must be specially designed for the safe and efficient production of sulfuric acid. In material selection, materials resistant to acid and high temperature conditions should be preferred. During the production phase of the equipment, it is of great importance to focus on criteria such as durability, efficiency and safety.
In addition, this process should also include modern production techniques such as environmentally friendly solutions and energy efficiency. Sulfuric acid plant equipment manufacturing is subjected to rigorous testing stages after assembly. These tests are a critical step in ensuring the performance and safety of the equipment. As a result, equipment manufactured for sulfuric acid plants are among the indispensable elements for the successful operation of industrial processes. Finally, continuous maintenance and control processes are vital to ensure the longevity of the equipment and the prevention of possible failures. Therefore, sulfuric acid plant equipment manufacturing is an area that requires expertise and experience.
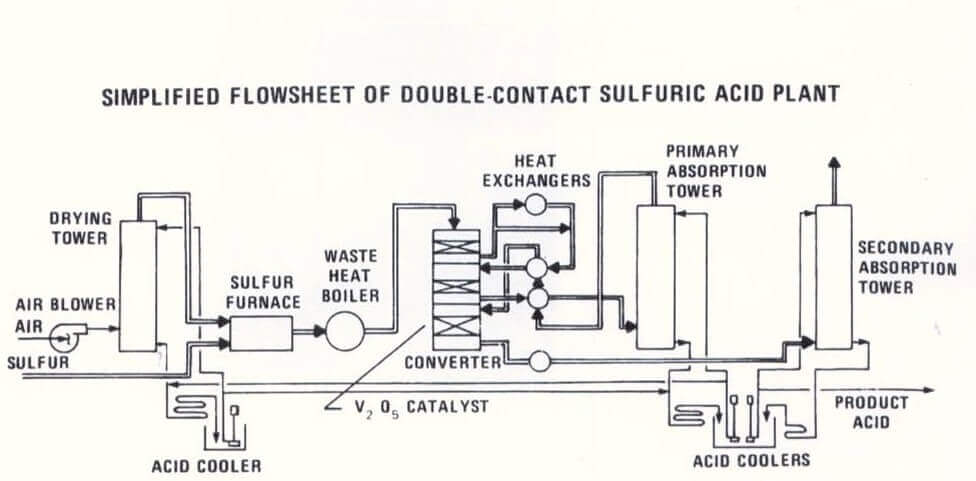
Sulfuric Acid Production Process
Sulfuric Acid is the most produced chemical in the world due to its widespread use in the Chemical, Metallurgical, Process, Petrochemical, Fertilizer industries as well as in the electrical and electronic, Semiconductor industries and also in various laboratories all over the world. Its most important area of use is in the phosphate industry and also in the metal extraction industries. They also find important uses in the Paint and Pigment, Explosive, Detergent, Plastic and Synthetic Fiber industries.
Sulfuric acid factory equipment manufacturing is a colorless liquid that resembles water. Its molecular weight is approximately 98. It is heavier than water. Sulfuric acid can be diluted with water to obtain acids of various strengths for different purposes. Sulfuric acid should be added to the water during the mixing process. This is because sulfuric acid is heavier than water and moves to the bottom due to gravity and the mixture is more homogeneous.
If we add water to sulfuric acid, water will remain on top because it is lighter than sulfuric acid. So the upper layer is water and the lower layer is sulfuric acid. At the interface, sulfuric acid will absorb water and release heat. This heat will vaporize some of the water near the interface. It will turn it into steam. The steam will expand and the mixture will explode and splash everywhere. Almost all of the sulfuric acid in the world is produced by the Contact Process.
Raw Materials Used in the Contact Process
Sulfuric acid factory equipment manufacturing consists of Sulfur, Oxygen and Hydrogen. Therefore, naturally, sources rich in these elemental compositions are selected for the production of Sulfuric Acid. The raw materials used can be elemental sulfur or Sulfur Dioxide or Pyrite. Air and water are also used. Sulfur can be found in mines, while Sulfur and Sulfur Dioxide can be found in flue gases obtained from coal or oil industries. Sulfur or Hydrogen Sulfide can be obtained from the desulfurization process of petroleum. Sulfur dioxide can be obtained from the smelting of metal silicide ores, as well as isolated from pyrite.
Sulfuric Acid Production Process
Sulfur Dioxide Gas (SO2) Production
Sulfur Trioxide Gas (SO3) Production
Sulfuric Acid (H2SO4) Production
Sulfuric acid equipment
The first step in the sulfuric acid plant equipment manufacturing process is the production of sulfur dioxide (SO2) by oxidation of an existing feedstock. A simplified flow diagram, Figure 1, presents three alternative routes for the production of SO2. The first is the combustion of sulfur; the second is the recovery of SO2 from metallurgical processes such as the roasting of pyrites and other sulfidic ores. The third alternative route is regeneration from spent acid.
Since such acids tend to be fouling, this process sequence usually begins with thermal degeneration, followed by washing of the resulting sulfur dioxide before cooling and drying. A catalytic converter, used to produce sulfur trioxide (SO3) from SO2, represents the most important part of a sulfuric acid plant. At approximately 425 °C (800 °F), SO2 reacts with excess air in the presence of vanadium pentoxide catalyst during several passes through catalyst beds inside the converter.
Since the reaction is highly exothermic, the gases are passed through gas-to-gas heat exchangers after each pass through the catalyst bed. SO3 (the anhydride of sulfuric acid) then enters the absorption towers where it reacts to form sulfuric acid. Approximately 35% of the heat generated in the process is used to raise the temperature of the sulfuric acid produced. In the temperature range of 155-190 °C (310-370 °F), relatively low corrosion rates have been found for stainless steels such as Type 304 (S30400) and Type 310 (S31000), duplex stainless steels such as Alloy 2205 (S32205), and high-silicon modified 5.5% Si stainless steels.
Material selection in sulfuric acid
Commercially concentrated sulfuric acid is one of the most important heavy industrial chemicals. Worldwide consumption is more than 200 million tons per year. It is sold primarily as 93%, 96%, and 98.5% H2SO4, but the “concentrated sulfuric acid” range nominally covers the range from 70% to 99%+. Lower acidities in the range of 25-70% are considered “medium” or “dilute” at 25%. The corrosion properties of these three concentration ranges are quite different, only the concentrated product shows the nature of a strong oxidizing acid.
This publication describes the behavior of nickel-containing alloys for Sulfuric acid plant equipment manufacturing and the special nature of their reactivity with both high and low concentrations, taken separately. The behavior of other metals commonly used in the production or processing of this acid is also discussed. Table 1 lists the nickel-containing alloys discussed in this introductory publication and their UNS numbers. It is not possible to mention all the alloys used in sulfuric acid here. A more detailed discussion of all aspects of material selection for sulfuric acid is available elsewhere
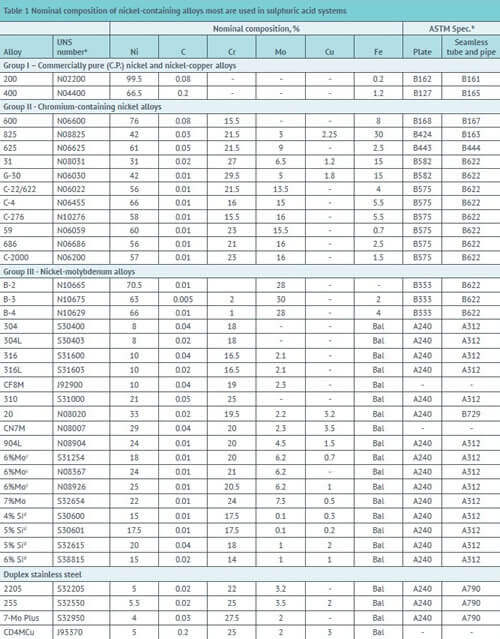
A – UNS numbers starting with “N” indicate a nickel alloy, but the definition of a nickel alloy is different from that used by ASTM.
B – In ASTM specifications, most nickel alloys fall into the “B” specifications. However, due to the redefinition of a nickel alloy, a few alloys such as 800 and 20 are being reclassified as stainless steels and will be included in the “A” specifications. This work is still ongoing. C – 6% MO alloys are a series of stainless steels, many of which are proprietary, all of which have roughly 6% MO content and are roughly equivalent.
D – High Si austenitic stainless steels are proprietary and were developed from nitric acid grades for use in strong sulfuric acid.
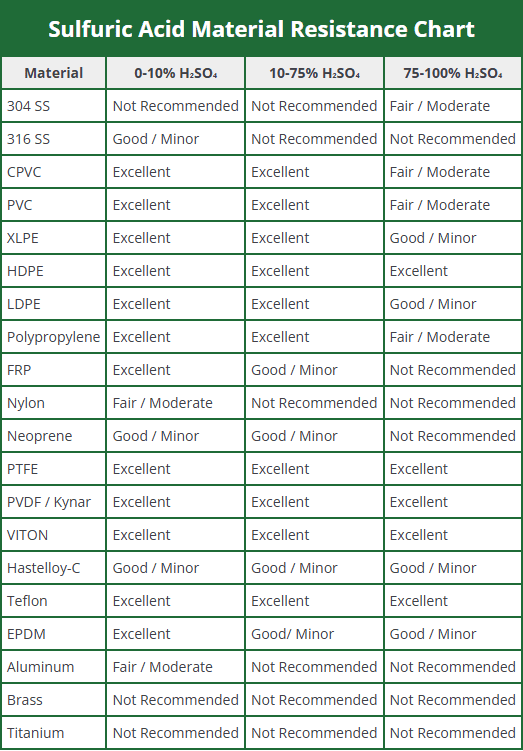
Sulphuric acid material resistance chart
When analyzing the material composition of storage tanks and related components in the manufacturing of sulfuric acid plant equipment, the concentration power of sulfuric acid must be taken into consideration. These containers are designed to conform to manufacturer specifications only within certain limits, depending on the properties of the chemical used and the concentration levels at which components may lose their performance. In this context, the table below highlights in detail the recommended bolts, gaskets and connection materials.